Laser-Lok® research
Laser-Lok research
Laser-Lok microchannels abstracts
Laser-Lok microchannels is a proprietary dental implant surface treatment developed from over 30 years of research initiated to create the optimal implant surface. Through this research, the unique Laser-Lok surface has been shown to elicit a biologic response that includes the inhibition of epithelial downgrowth and the attachment of connective tissue.1,2 This physical attachment produces a biologic seal around the implant that protects and maintains crestal bone health. The Laser-Lok phenomenon has been shown in post-market studies to be more effective than other implant designs in reducing bone loss.3,4,5,6
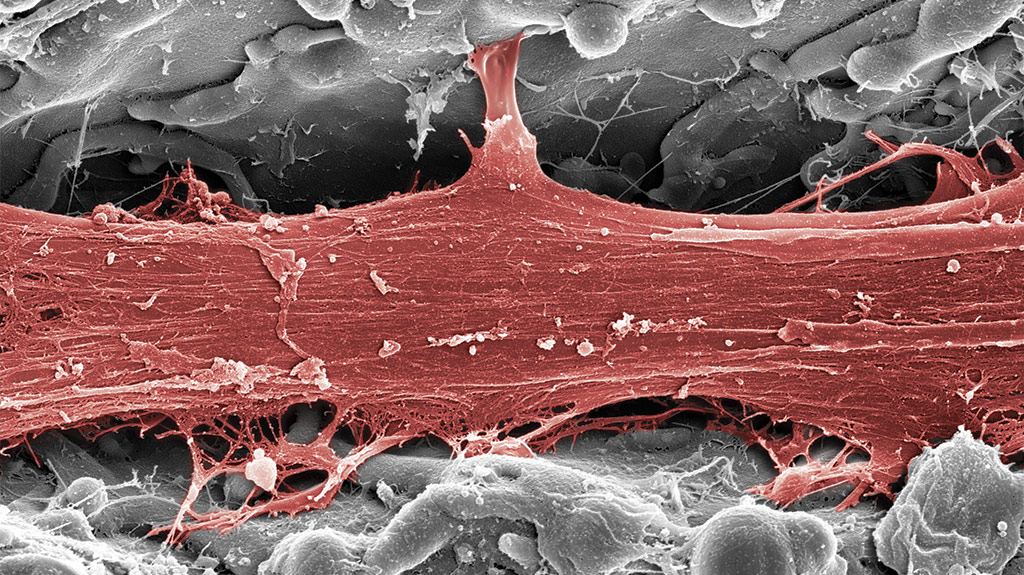
latest implant research
Objectives
To assess the clinical, radiographic, and esthetic outcomes of implants with a laser microtextured collar placed in the anterior region of the maxilla at the time of tooth extraction and immediately temporized.
Methods
Forty-six Tapered Internal Laser-Lok BioHorizons implants were immediately placed and immediately restored with nonfunctional loading in 46 patients (24 men and 22 women) with a thick gingival biotype, ideal gingival level/contour, and postextraction intact walls. Survival rate, cortical bone loss, and periimplant mucosal responses were evaluated at 6, 12, and 24 months.
Results
Survival rate was 95.6%. Mean mesial and distal marginal bone loss, 24 months after installation, were 0.58 mm (SD = 0.53; range, 0.17–1.15) and 0.57 mm (SD = 0.70; range, 0.42–1.10), respectively. A mesial and distal papilla regrowth mean of 1.8 and 1.5 mm, respectively, were found. The midfacial soft tissue levels showed 0.12 mm of mean recession after 24 months.
Conclusion
Immediate implants with a laser microtextured surface restored at the day of surgery, may be considered as a predictable procedure in terms of implant survival and hard and soft tissue remodeling.
ABSTRACT
Interimplant papillae are critical to achieving esthetic implant-supported restorations in the maxillary esthetic zone. Stable papillary anatomy, however, depends upon a stable volume of underlying crestal bone for support. Multiple studies have documented a critical interimplant distance of 3 mm, under which crestal bone resorption occurs. The current preclinical proof-of-principle canine study examines a novel implant-abutment system design, combining platform switching with precisely configured laser-ablated abutment and implant microgrooves to maintain interimplant crestal bone at interimplant distances of 2 and 4 mm. Results of this initial preclinical study suggest that it is possible through precise implant/abutment design modifications to place adjacent implants at distances of 2 to 4 mm without inducing subpapillary crestal bone loss.
SOFT TISSUE FINDINGS
Peri-implant soft tissues consisted of an epithelial barrier, with the sulcular epithelium merging with the junctional epithelium. The junctional epithelium ended abruptly at the coronal-most position of the abutment Laser-Lok microgrooves, where a zone of CT fibers appeared to enter perpendicularly into the microchanneled 0.7-mm tall band. In addition, CT fibers also appeared to enter into Laser-Lok regions of the implant collar, effectively sealing the IAJ microgap from surrounding tissues. Importantly, no evidence of an inflammatory infiltrate was found in any specimen at the IAJ.
HARD TISSUE FINDINGS
Interimplant crestal bone showed no evidence of bone resorption in any biopsy specimen at the end of 3 months. Significant bone-to-implant contact (BIC) was readily apparent along all aspects of the implant body and collar. In many specimens, regenerated bone was seen immediately proximal to the IAJ microgap. The apposition of both perpendicularly inserting CT fibers and bone onto the laser-ablated microchannels in the region of the IAJ microgap served to anatomically seal the IAJ from surrounding tissues and prevent migration of the junctional epithelium.
INTRODUCTION
A physical attachment of connective tissue fibers to the laser microtexturing (8 and 12μm grooves) surface placed on collar of implant, has been demonstrated using human histology. Related clinical research has suggested that this kind of microtexturing surface may lead to a decreased amount of initial bone loss.
AIM
The aim of this retrospective study was to compare crestal bone heights and clinical parameters between implants with laser-microtextured collar and machined collar using different protocols.
MATERIALS AND METHODS
This study evaluates 300 single implants in 300 patients (155 males and 145 females; mean age: 49.3 years; range: 45 to 75 years). 160 implants with laser-microtextured collars (L) and 140 with machined collars (M) were used. Implants were grouped into the treatment categories of immediate placement, delayed placement, immediate non-occlusal loading (INOL), and delayed loading (DL). For all groups, crestal bone level (CBL), attachment level (CAL), plaque index (PI), and bleeding on probing (BOP), were recorded at baseline examinations (BSL) and 6 (T1), 12 (T2), and 24 months (T3) after loading with the final restoration.
RESULTS
Nine implants were lost (four L and five M). The type of implant and time of placement and loading showed no significant influence on the survival rate. A mean CAL loss of 1.12 mm was observed during the first 2 years in the M group, while the mean CAL loss observed in L the group was 0.55 mm. Radiographically, L group implants showed a mean crestal bone loss of 0.58 mm compared to 1.09 mm for the M.
CONCLUSIONS
Results suggest that laser microtextured surface on implant collar may mitigate the negative sequelae connected with the peri-implant bone loss regardless of the type of positioning and loading protocol used.
BACKGROUND
Recently, new implant surfaces have been proposed in an effort to improve hard and soft tissue integration, which may be beneficial in immediate loading situations.
AIM
The purpose of the present prospective clinical study was to, during 2 years, clinically and radiographically evaluate an implant with laser microtextured collar surface placed for immediate loading of fixed prostheses in cases of partial posterior maxillary and/or mandibular edentulism.
MATERIALS AND METHODS
Thirty-five partially edentulous patients who needed implant treatment and met inclusion criteria were consecutively enrolled at different study centers in Italy. A total of 107 Tapered Internal Laser-Lok implants (49 maxillary and 58 mandibular) were placed and immediately loaded. All provisional constructions were delivered within 1 hour, and the final constructions placed after 4 months. A total of 32 prosthetic constructions, consisting of 10 two-units, 12 three-units, and 10 four-units restorations, were evaluated. Implants were monitored for clinical and radiographic outcomes at follow-up examinations scheduled for 6, 12, 24 months.
RESULTS
Five implants have been lost after loading (3 implants in a two-unit maxillary restorations, 1 implant in a two-unit mandibular restoration, and 1 implant in three-unit maxillary restoration) giving a survival rate of 95.4% after 24 months. Mean marginal bone loss 6,12, and 24 months after installation was of 0.42mm ± 1.1mm, 0.52mm ± 0.9mm, and 0.66mm ±1.3 mm, respectively.
CONCLUSIONS
Although limited to the short follow-up, immediate function with Tapered Internal Laser-Lok® implants seems to be a viable option to treated partially edentulous patients.
ABSTRACT
The purpose of the present clinical study was to evaluate the influence of Laser-Lok® microtexturing surface on clinical attachment level and crestal bone remodeling around immediate functional loaded implants in single-tooth replacement in the area of 15-25 and 35-45.
MATERIALS AND METHODS
Seventy-seven patients were included in a prospective, randomized study and divided in two groups: in the control group BioHorizons Tapered Internal non-Laser-Lok®-type (NLL; n=39) implants were used, while in test group BioHorizons Tapered Internal Laser-Lok®-type (LL; n=39) was used. Crestal bone loss (CBL), and clinical parameters including clinical attachment level (CAL), plaque index (PI), and bleeding on probing (BOP), were recorded at baseline examinations (BSL) and 6 (T1), 12 (T2), and 24 months (T3) after loading with the final restoration.
RESULTS
One implants was lost in the control group, and one in the test group, giving a total survival rate of 96,1% after 2 years. PI and BOP outcomes were found similar for both implant types without statistical differences. A mean CAL loss of 1.10mm ± 0.51mm was observed during the first 2 years in the NLL group, while the mean CAL loss observed in LL the group was 0.56mm ± 0.33mm. Radiographically, NLL group implants showed a mean crestal bone loss of 1.07mm ± 0.30mm compared to 0.49mm ± 0.34mm for the LL.
CONCLUSIONS
The type of implants did not influence the survival rate, whereas LL resulted in greater CAL and in shallower radiographic peri-implant CBL than NLL.
ABSTRACT
Histological and clinical studies confirm that laser microtexturing of implant collars favors the attachment of connective fibers and reduces probing depth and peri-implant bone loss, when compared to machined collars. This prospective study aimed at assessing the alveolar dimensional changes after immediate transmucosal implants placement (Laser-Lok® microtextured collar) associated with bone regenerative procedures.
MATERIALS AND METHODS
Thirteen implants (Single-Stage Implant System®, BioHorizons, IPH. Inc.) were placed immediately into single-rooted extraction sockets. Periimplant defects were treated with bovine-derived xenografts (Laddec®, BioHorizons, IPH. Inc) and resorbable collagen membranes (Mem-Lok®, BioHorizons, IPH. Inc.).
RESULTS
At 6-months surgical re-entry, Laser-Lok® microtextured collar provides more favorable conditions for the attachment of hard and soft tissues, and reduces the alveolar bone loss.
additional information
- Human Histologic Evidence of a Connective Tissue Attachment to a Dental Implant.
M Nevins, ML Nevins, M Camelo, JL Boyesen, DM Kim. International Journal of Periodontics & Restorative Dentistry. Vol. 28, No. 2, 2008. - The Effects of Laser Microtextured Collars Upon Crestal Bone Levels of Dental Implants.
S Weiner, J Simon, DS Ehrenberg, B Zweig, JL Ricci. Implant Dentistry, Volume 17, Number 2, 2008. p. 217-228 - Clinical Evaluation of Laser Microtexturing for Soft Tissue and Bone Attachment to Dental Implants.
Pecora GE, Ceccarelli R, Bonelli M, Alexander H, Ricci JL. Implant Dent. 2009 Feb;18(1):57-66. - The Effects of Laser Microtexturing of the Dental Implant Collar on Crestal Bone Levels and Peri-implant Health.
Botos S, Yousef H, Zweig B, Flinton R, Weiner S. Int J Oral Maxillofac Implants. 2011 May-Jun;26(3):492-8. - Radiographic Analysis of Crestal Bone Levels on Laser-Lok® Collar Dental Implants.
CA Shapoff, B Lahey, P Wasserlauf, D Kim. Int J Periodontics Restorative Dent 2010;30:129-137. - Marginal Tissue Response to Different Implant Neck Design.
HEK Bae, MK Chung, IH Cha, DH Han. J Korean Acad Prosthodont. 2008, Vol. 46, No. 6.
human studies
INTRODUCTION
A tapered dental implant (Laser-Lok [LL] surface treatment) with a 2 mm wide collar, that has been laser micromachined in the lower 1.5 mm to preferentially accomplish bone and connective tissue attachment while inhibiting epithelial downgrowth, was evaluated in a prospective, controlled, multicenter clinical trial.
MATERIALS
Data are reported at measurement periods from 1 to 37 months postoperative for 20 pairs of implants in 15 patients. The implants are placed adjacent to machined collar control implants of the same design. Measurement values are reported for bleeding index, plaque index, probing depth, and crestal bone loss.
RESULTS
No statistical differences are measured for either bleeding or plaque index. At all measurement periods there are significant differences in the probing depths and the crestal bone loss differences are significant after 7 months (P<0.001). At 37 months the mean probing depth is 2.30 mm and the mean crestal bone loss is 0.59 mm for LL versus 3.60 and 1.94 mm, respectively, for control implant. Also, comparing results in the mandible versus those in the maxilla demonstrates a bigger difference (control implant - LL) in the mean in crestal bone loss and probing depth in the maxilla. However, this result was not statistically significant.
DISCUSSION
The consistent difference in probing depth between LL and control implant demonstrates the formation of a stable soft-tissue seal above the crestal bone. LL limited the crestal bone loss to the 0.59 mm range as opposed to the 1.94 mm crestal bone loss reported for control implant. The LL implant was found to be comparable with the control implant in safety endpoints plaque index and sulcular bleeding index. There is a nonstatistically significant suggestion that the LL crestal bone retention superiority is greater in the maxilla than the mandible.
ABSTRACT
Purpose: Polished and machined collars have been advocated for dental implants to reduce plaque accumulation and crestal bone loss. More recent research has suggested that a roughened titanium surface promotes osseointegration and connective tissue attachment. The purpose of this research was to compare crestal bone height adjacent to implants with laser-microtextured and machined collars from two different implant systems (Laser-Lok and Nobel Replace Select).
Materials and Methods
Four implants, two Laser-Lok and two Nobel Replace Select, were placed in the anterior mandible to serve as overdenture abutments. They were placed in alternating order, and the distal implants were loaded with ball abutments. The mesial implants were left unloaded. The distal implants were immediately loaded with prefabricated dentures. Plaque Index, Bleeding Index, and probing depths (PDs) were measured after 6 and 12 months for the loaded implants. Bone loss for both groups (loaded and unloaded) was evaluated via standardized radiographs.
Results
Plaque and bleeding values were similar for both implant types. The Laser-Lok implants showed shallower PDs (0.36 ± 0.5 mm and 0.43 ± 0.51 mm) than those Nobel Replace Select (1.14 ± 0.77 mm and 1.64 ± 0.93 mm; P < .05 for 6 and 12 months, respectively). At 6 and 12 months, respectively, the Laser-Lok implants showed less crestal bone loss for both loaded (0.19 ± 0.15 mm and 0.42 ± 0.34 mm) and unloaded groups (0.15 ± 0.15 mm and 0.29 ± 0.20 mm) than the Nobel Replace Select implants for both the loaded (0.72 ± 0.5 mm and 1.13 ± 0.61 mm) and unloaded groups (0.29 ± 0.28 mm and 0.55 ± 0.32 mm).
Conclusion
Laser-Lok implants resulted in shallower PDs and less peri-implant crestal bone loss than that seen around Nobel Replace Select.
Restorations for Cases 1 and 2 by Jeffrey A. Babushkin, DDS (Trumbull, CT)
Restoration for Case 3 by Dr. Perry Kest (Southbury, CT)
ABSTRACT
Numerous published animal and human dental implant studies report crestal bone loss from the time of placement of the healing abutment to various time periods after restoration. The bone loss can result in loss of interproximal papilla and recession of crown margins. These three case reports demonstrate the long-term results that can be obtained utilizing implants with the Laser-Lok microchannel collar design to preserve crestal bone and soft tissue esthetics. Case 1 involved extraction, socket grafting, 6 month delayed implant placement and final restoration in 6 months. Case 2 involved extraction, immediate implant placement with simultaneous grafting and provisional crown placement two months later. Case 3 involved treatment of congenitally missing laterals with a delayed restoration.
Case 1 (first reported use of a Laser-Lok implant)
A 34 year old female presented with external resorption at the level of the CEJ of tooth #9. Various treatment options were presented and the patient elected extraction and dental implant placement. After atraumatic extraction, the socket anatomy did not allow for immediate placement with acceptable initial stability. The socket was grafted with allograft calcified bone and allowed to heal for 6 months. At that time a dental implant with Laser-Lok microchannel collar design was placed. A subepithelial connective tissue graft was also utilized on the adjacent tooth #10 for root coverage. Six months after placement second stage surgery was performed and the tooth was restored with a customized abutment and PFM crown. Note the maintenance of excellent crestal bone levels (within 0.5mm of the implant/abutment interface) at 13 years post-restoration. The soft tissue margins have remained stable and exhibit excellent periodontal health.
Case 2
This 60 year old female presented with chronic infection evidenced by a fistula at the apical extent of tooth #9. This tooth had previously been treated with root canal and apical surgery. All treatment options were reviewed with the patient and dental implant replacement was chosen. Because the patient was relocating to South America for a period of two years, immediate extraction, dental implant placement with socket grafting was performed. The dental implant was a 5 mm x 13 mm Laser-Lok microchannel collar design. A provisional crown was placed 2 months following implant placement. The patient had no other professional dental care for two years and upon returning home, the final crown was placed. Note the crestal bone levels (within 0.5 mm of the abutment/implant interface) at four years after implant loading.
Case 3
A 17 year old female with congenitally missing maxillary lateral incisors was referred for dental implant consideration in both sites. Following a clinical examination including a CAT scan radiographic study, a surgical procedure was performed involving esthetic crown lengthening from tooth #4 to #13 and placement of BioHorizons Tapered Internal Implants 3.8 mm x 12mm (3.5mm platform) in sites #7 and #10. Utilizing a surgical guide, the implants were placed with the implant collar 2-3 mm from the intended facial gingival margin of the planned crowns. Only 0.3 mm of the machined metal collar was exposed on the mid-facial surface. Healing was uneventful. Second stage surgery and placement of healing abutments was performed 4 months after initial surgery.
Conclusion
These three cases demonstrate the ability of the Laser-Lok microchannel collar design to maintain crestal bone levels and soft tissue esthetics around dental implants. Two cases involved implant placement in grafted sites. All three cases demonstrate unequivocal clinical and radiographic evidence of crestal bone stability in close proximity to the abutment/implant interface (micro-gap). A traditional expectation of bone loss below the collar and to the first thread was not noted. The ability of the Laser-Lok microchannels to maintain crestal bone and provide supracrestal connective tissue attachment may create a new definition of "normal" implant biologic width.
ABSTRACT
This human proof-of-principle study was designed to investigate the possibility of achieving a physical connective tissue attachment to the Laser-Lok microchannel collar of a dental implant. Its 2-mm collar has been micromachined to encourage bone and connective tissue attachment while preventing apical migration of the epithelium. Implants were harvested with the surrounding implant soft and hard tissues after 6 months. The histologic investigation was conducted with light microscopy, polarized light, and scanning electron microscopy.
RESULTS
The implants were osseointegrated with histologic evidence of direct bone contact. There was a connective tissue attachment to the Laser-Lok microchannels. There were no signs of inflammation. The peri-implant tissues consisted of a dense, collagenous lamina propria covered with a stratified, squamous, keratinizing oral epithelium. The latter was continuous with the parakeratinized sulcular epithelium that lined that lateral surface of the peri-implant sulcus. Apically, the sulcular epithelium overlapped the coronal border of the junctional epithelium. The sulcular epithelium was continuous with the junctional epithelium, which provided epithelial union between the implant and the surrounding peri-implant mucosa. Between the apical termination of the junctional epithelium and the alveolar bone crest, connective tissue directly apposed the implant surface.
Light microscopic evaluation of these specimens revealed intimate contact of the junctional epithelial cells with the implant surface. The microgrooved area of the implants was covered with connective tissue. Polarized light microscopy of this area revealed functionally oriented collagen fibers running toward the grooves of the implant surface. SEM of a corresponding area of the specimen confirmed the presence of attached collagen fibers.
All specimens demonstrated a high degree of bone-to-implant contact and intense remodeling activity. In specimens that showed collagen fibers functionally oriented toward the grooves on the implant surface, remodeling of new bone in the coronal direction was observed. SEM revealed sulcular epithelium with the desquamating activity of the cells and the junctional epithelium. It appears that the connective tissue attachment is instrumental in preserving the alveolar bone crest and inhibiting apical migration of the epithelium.
ABSTRACT
This human proof-of-principle study was designed to investigate the possibility of achieving a physical connective tissue attachment to the Laser-Lok microchannel collar of a dental implant. Its 2-mm collar has been micromachined to encourage bone and connective tissue attachment while preventing apical migration of the epithelium. Implants were harvested with the surrounding implant soft and hard tissues after 6 months. The histologic investigation was conducted with light microscopy, polarized light, and scanning electron microscopy.
RESULTS
The implants were osseointegrated with histologic evidence of direct bone contact. There was a connective tissue attachment to the Laser-Lok microchannels. There were no signs of inflammation. The peri-implant tissues consisted of a dense, collagenous lamina propria covered with a stratified, squamous, keratinizing oral epithelium. The latter was continuous with the parakeratinized sulcular epithelium that lined that lateral surface of the peri-implant sulcus. Apically, the sulcular epithelium overlapped the coronal border of the junctional epithelium. The sulcular epithelium was continuous with the junctional epithelium, which provided epithelial union between the implant and the surrounding peri-implant mucosa. Between the apical termination of the junctional epithelium and the alveolar bone crest, connective tissue directly apposed the implant surface.
Light microscopic evaluation of these specimens revealed intimate contact of the junctional epithelial cells with the implant surface. The microgrooved area of the implants was covered with connective tissue. Polarized light microscopy of this area revealed functionally oriented collagen fibers running toward the grooves of the implant surface. SEM of a corresponding area of the specimen confirmed the presence of attached collagen fibers.
All specimens demonstrated a high degree of bone-to-implant contact and intense remodeling activity. In specimens that showed collagen fibers functionally oriented toward the grooves on the implant surface, remodeling of new bone in the coronal direction was observed. SEM revealed sulcular epithelium with the desquamating activity of the cells and the junctional epithelium. It appears that the connective tissue attachment is instrumental in preserving the alveolar bone crest and inhibiting apical migration of the epithelium.
BACKGROUND
The purpose of this study is to assess the influence of the placement level of implants with a laser-microtextured collar design on the outcomes of crestal bone and soft tissue levels. In addition, we assessed the vertical and horizontal defect fill and identified factors that influenced clinical outcomes of immediate implant placement.
METHODS
Twenty-four patients, each with a hopeless tooth (anterior or premolar region), were recruited to receive dental implants. Patients were randomly assigned to have the implant placed at the palatal crest or 1mm subcrestally. Clinical parameters including the keratinized gingival (KG) width, KG thickness, horizontal defect depth (HDD), facial and interproximal marginal bone levels (MBLs), facial threads exposed, tissue-implant horizontal distance, gingival index (GI), and plaque index (PI) were assessed at baseline and 4 months after surgery. In addition, soft tissue profile measurements including the papilla index, papilla height (PH), and gingival level (GL) were assessed after crown placement at 6 and 12 months post-surgery.
Results
The overall 4-month implant success rate was 95.8% (one implant failed). A total of 20 of 24 patients completed the study. At baseline, there were no significant differences between crestal and subcrestal groups in all clinical parameters except for the facial MBL (P = 0.035). At 4 months, the subcrestal group had significantly more tissue thickness gain (keratinized tissue) than the crestal group compared to baseline. Other clinical parameters (papilla index, PH, GL, PI, and GI) showed no significant differences between groups at any time. A facial plate thickness <=1.5 mm and HDD >=2 mm were strongly correlated with the facial marginal bone loss. A facial plate thickness <=2 mm and HDD >=3 were strongly correlated with horizontal dimensional changes.
Conclusion
The use of immediate implants was a predictable surgical approach (96% survival rate), and the level of placement did not influence horizontal and vertical bone and soft tissue changes. This study suggests that a thick facial plate, small gaps, and premolar sites were more favorable for successful implant clinical outcomes in immediate implant placement.
ABSTRACT
Objective: To perform a histologic and histomorphometric analysis of the peri-implant tissue reactions and bone-titanium interface in 3 immediately loaded (provisional loaded) titanium implants retrieved from a man after a loading period of 4 months.
Materials & Methods: A 35 year-old patient with a maxillary partial edentulism did not want to wear a provisional removable prosthesis during the healing period. It was decided to insert 3 definitive implants and use 3 provisional implants for the transitional period. The provisional implants were loaded the same day with a resin prosthesis in occlusal contact. During the second surgical phase, after 4 months, the provisional prosthesis was removed, and the provisional implants were retrieved with a trephine bur. Before retrieval, all implants appeared to be clinically osseointegrated. The specimens were processed for observation under light microscopy.
Results: At low magnification, it was possible to observe that bone trabeculae were present around the implant. Areas of bone remodeling and haversian systems were present near the implant surface. Under polarized-light microscopy, it was possible to observe that in the coronal aspect of the thread, the lamellar bone showed lamellae that tended to run parallel to the implant surface, while in the inferior aspect of the thread, the bone lamellae ran perpendicular to the implant surface.
ABSTRACT
Little is known about the in vivo healing processes at the interface of implants placed in different grafting materials. For optimal sinus augmentation, a bone graft substitute that can regenerate high-quality bone and enable the osseointegration of load-bearing titanium implants is needed in clinical practice. Calcium sulphate (CaS) is one of the oldest biomaterials used in medicine, but few studies have addressed its use as a sinus augmentation material in conjunction with simultaneous implant placement. The aim of the present study was to histologically evaluate an immediately loaded provisional implant retrieved 7 months after simultaneous placement in a human sinus grafted with CaS. During retrieval, bone detached partially from one of the implants which precluded its use for histologic analysis. The second implant was completely surrounded by native and newly formed bone, and it underwent histologic evaluation. Lamellar bone, with small osteocyte lacunae, was present and in contact with the implant surface. No gaps, epithelial cells, or connective tissues were present at the bone-implant interface. No residual CaS was present. Bone-implant contact percentage was 55% ± 8%. Of this percentage, 40% was represented by native bone and 15% by newly formed bone. CaS showed complete resorption and new bone formation in the maxillary sinus; this bone was found to be in close contact with the implant surface after immediate loading.
ABSTRACT
Endosseous dental implants have traditionally been placed using a two-stage surgical procedure with a 6- to 12-month healing period following tooth extraction. In order to decrease healing time, protocols were introduced that included immediate implant placement and provisionalization following tooth extraction. Although survival rates for this technique are high, postoperative gingival shrinkage and bone resorption in the aesthetic zone are potential limitations. The two case reports described herein present a surgical technique for the preservation of anterior aesthetics that combines minimally invasive extraction, immediate implant placement, provisionalization, and the use of implants with a laser micro-grooved coronal design.
DISCUSSION
The use of implants with a laser microgrooved coronal design may have contributed to the maintenance of buccal soft tissue, providing attachment and preventing epithelial cell downgrowth, which often occurs with machined collar implants. Maintenance of this supra crestal soft tissue often depends on its ability to establish an attachment supercrestally to the implant surface.
latest abutment research
ABSTRACT
Previous research has demonstrated the effectiveness of laser-ablated microgrooves placed within implant collars in supporting direct connective tissue attachments to altered implant surfaces. Such a direct connective tissue attachment serves as a physiologic barrier to the apical migration of the junctional epithelium (JE) and prevents crestal bone resorption. The current prospective preclinical trial sought to evaluate bone and soft tissue healing patterns when laser-ablated microgrooves are placed on the abutment. A canine model was selected for comparison to previous investigations that examined the negative bone and soft tissue sequelae of the implant-abutment microgap. The results demonstrate significant improvement in peri-implant hard and soft tissue healing compared to traditional machined abutment surfaces.
MATERIALS AND METHODS
The current study was designed to examine the effects of two different implant and abutment surfaces on epithelial and connective tissue attachment, as well as peri-implant bone levels. Six foxhounds were selected for this study. Each dog received 6 implants in the bilateral mandibular premolar and first molar extraction sites, for a total of 36 implants. The sites were randomly assigned to receive tapered internal implants (BioHorizons) with either resorbable blast texturing (RBT) or RBT with a 0.3mm machined collar. In addition, either machined-surface or Laser-Lok microchannel healing abutments were assigned randomly to each implant. The abutments were placed at the time of surgery.
RESULTS
The presence of the 0.7 mm laser ablated microchanneled zone consistently enabled intense fibroblastic activity to occur on the abutment grooved surface, resulting in a dense interlacing complex of connective tissue fibers oriented perpendicular to the abutment surface that served as a physiologic barrier to apical JE migration. As a consequence of inhibiting JE apical migration, crestal bone resorption was prevented. Significantly, in two cases bone regeneration coronal to the IAJ and onto the abutment surface occurred, completely eliminating the negative sequelae of the IAJ microgap.
In contrast, abutments devoid of laser-ablated microgrooved surfaces, exhibited little evidence of robust fibroblastic activity at the abutment-tissue interface. A long JE extended along the abutment and implant collar surfaces, preventing formation of the physiologic connective tissue barrier and causing crestal bone resorption. Parallel rather than functionally oriented perpendicular connective tissue fibers apposed the abutment implant surfaces.
INTRODUCTION
Human histology and scanning electron microscopy (SEM) are presented, outlining the soft tissue integration to a laser microgrooved abutment surface.
CASE PRESENTATION
In two patients, prosthetic abutments with a laser microgrooved surface were placed on osseointegrated implants. After 6 weeks of healing, the abutments and the surrounding soft tissue were removed and prepared for histology and SEM. The most apical epithelium was found coronal to this surface. Connective tissue demonstrated collagen fibers oriented perpendicular to the microgrooved surface. There was intimate contact between the connective tissues and the microgrooved abutment surface.
CONCLUSION
The abutments in these patients had connective tissue integration with functionally oriented fibers to the microgrooved surface.
SUMMARY
Why is this case new information? To our knowledge, this is the first human case series reported with human histology describing the connective tissue attachment around a microgrooved abutment.
What are the keys to successful management in this case? The abutment surface characteristics can lead to connective tissue integration with the microgrooved surface, with functionally oriented collagen fibers.
What are the primary limitations to success in this case? This is only a case series of the histology of the attachment. No clinical outcomes or advantages are reported. Further studies will need to be conducted to demonstrate the clinical advantages.
ABSTRACT
Previous preclinical and clinical studies have demonstrated the effectiveness of precisely configured laser-ablated microgrooves placed on implant collars to allow direct connective tissue attachment to the implant surface. A recent canine study examining laser-ablated microgrooves placed in a defined healing abutment area demonstrated similar findings. In both instances direct connective tissue attachment to the implant/abutment surface served as an obstacle to the apical migration of the junctional epithelium, thus preventing crestal bone resorption. The current case report examined the effectiveness of abutment positioned laser-ablated microgrooves in human subjects. As in the preclinical trial, precisely defined laser-ablated microgrooves allowed direct connective tissue attachment to the altered abutment surface, prevented apical migration of the junctional epithelium and thus protected crestal bone from premature resorption.
ABSTRACT
This report presents human evidence of reattachment of the connective tissue when the laser microgrooved healing abutment has been replaced by the laser microgrooved cylindric permanent abutment. No additional bone loss has been noted 15 weeks after the placement of the laser microgrooved cylindric permanent abutment. A dense connective tissue was in intimate contact with the laser microgrooved surface to the point of the soft tissue separation, and a clear evidence of the junctional epithelium ending at the coronal-most position of the laser microgrooved zone was identified.
OBJECTIVES
To (i) investigate the influence of different extensions of a laser microgrooved abutment zone on connective tissue attachment and (ii) assess the impact of a repeated abutment dis-/reconnection on soft- and hard-tissue healing.
MATERIALS AND METHODS
Titanium implants were inserted epicrestally in the lower jaws of six dogs. Healing abutments with either partially (LP) or completely (LC) laser microgrooved margins or machined surface margins (M) were randomly allocated either to a single (1×)/repeated (2×) dis-/reconnection at 4 and 6 weeks (test), respectively, or left undisturbed (control). At 6 and 8 weeks, histomorphometrical (e.g. most coronal level of bone in contact with the implant [CBI], subepithelial connective tissue attachment [STC]) and immunohistochemical (Collagen Type-I [CI]) parameters were assessed.
RESULTS
At control sites, LP/LC groups revealed lower mean CBL (8 weeks, 0.95 ± 0.51 vs. 0.54 ± 0.63 vs. 1.66 ± 1.26 mm), higher mean STC (8 weeks, 82.58 ± 24.32% vs. 96.37 ± 5.12% vs. 54.17 ± 8.09%), but comparable CI antigen reactivity. A repeated abutment manipulation was associated with increased mean CBL (8 weeks, 1.53 ± 1.09 vs. 0.94 ± 0.17 vs. 1.06 ± 0.34 mm), decreased STC (8 weeks, 57.34 ± 43.06% vs. 13.26 ± 19.04% vs. 37.76 ± 37.08%) and CI values.
CONCLUSIONS
It was concluded that (i) LC>LP abutments enhanced subepithelial connective tissue attachment and preserved crestal bone levels, (ii) repeated abutment dis-/reconnection during the initial healing phase (4-6 weeks) may be associated with increased soft- and hard-tissue changes and (iii) LP and LC should be considered using a one abutment, one time approach.
additional pre-clinical research
ABSTRACT
This paper summarizes current knowledge on the benefits of laser-ablated microgrooves in neck regions of endosseous dental implants. Like machine-tooled coronal microthreads with particle-blasted surfaces, laser-ablated microgrooves help to preserve crestal bone. However, they also appear to uniquely favor a true gingival connective tissue attachment comparable to that of natural teeth.
Materials and Methods: A literature search of publications in refereed journals in the English language from 1990 to July 2011 was performed using the National Library of Medicine and SCOPUS Cochrane Oral Health Group databases. Additional papers from reference lists of identified papers, but preceding 1990, were also reviewed. Relevant references were selected on the basis of titles and abstracts, but final selections were based on full-text review independently by the two authors.
Conclusion: Dental implants with laser-ablated coronal microgrooves or particle-blasted machine-tooled microthreads reduce peri-implant crestal bone loss compared to implants with fully machine-turned or particle-blasted (without the addition of microthreads) collar segments. However, unlike machine-tooled microthreads, laser microgrooves appear to inhibit apical migration of crevicular epithelium and promote true attachment of peri-implant gingiva. Since both treatments result in similar surface roughness, the difference in response of connective tissue may relate to differences in nanotopography and the fact that laser microgrooves are an order of magnitude smaller in dimension than machine-tooled microthreads. It can be speculated that formation of a connective tissue–implant collar interface more like that of a natural tooth will improve long-term performance of dental implants.
ABSTRACT
Objective: This study compared the alveolar bone reduction after immediate implantation using microgrooved and smooth collar implants in fresh extracted sockets.
Materials and Methods: Four mongrel dogs were used in this study. The full buccal and lingual mucoperiosteal flaps were elevated and the third and fourth premolars of the mandible were removed. The implants were installed in the fresh extracted sockets. The animals were sacrificed after a 3-month healing period. The mandibles were dissected and each implant site was removed and processed for a histological examination.
Results: During healing, the marginal gaps in both groups, which were present between the implant and the socket walls at implantation, disappeared as a result of bone filling and resorption of the bone crest. The buccal bone crests were located apical of its lingual counterparts. At the 12-week interval, the mean bone-implant contact in the microgrooved collar group was significantly higher than that of the turned collar group. From the observations in some of the microgrooved collar groups, we have found bone attachment to the 12 µm microgrooved surface and collagen fibers perpendicular to the long axis of the implants over the 8 µm microgrooved surface.
Conclusion: Within the limitations of this study, microgrooved implants may provide more favorable conditions for the attachment of hard and soft tissues and reduce the level of marginal bone resorption and soft tissue recession.
ABSTRACT
This study, analytically, through finite element analysis, predicts the minimization of crestal bone stress resulting from implant collar surface treatment. A tapered dental implant design with Laser-Lok (LL) and without (control, C) laser microgrooving surface treatment are evaluated. The LL implant has the same tapered body design and thread surface treatment as the C implant, but has a 2-mm wide collar that has been laser micromachined with 8 and 12μm grooves in the lower 1.5 mm to enhance tissue attachment. In vivo animal and human studies previously demonstrated decreased crestal bone loss with the LL implant. Axial and side loading with two different collar/bone interfaces (nonbonded and bonded, to simulate the C and LL surfaces, respectively) are considered. For 80 N side load, the maximum crestal bone distortional stress around C is 91.9 MPa, while the maximum crestal bone stress around LL, 22.6 MPa, is significantly lower. Finite element analysis suggests that stress overload may be responsible for the loss of crestal bone. Attaching bone to the collar with LL is predicted to diminish this effect, benefiting crestal bone retention.
Yonsei University College of Dentistry, Seoul, South Korea
ABSTRACT
Purpose: This animal study examined the histomorphometric variations between a turned neck (TN) implant with a RBM body, a microthreaded (MT) neck implant and a micro-grooved (MG) implant (Laser-Lok).
Materials and Methods: Mandibular premolars from four mongrel dogs were removed and left to heal for three months. One of three harvested for histological examination. All specimens have shown uneventful healing for the duration of the experiment.
Results: The histological slides have shown that all samples osseointegrated successfully with active bone remodeling adjacent to the implants. With the Laser-Lok implants, 0.40mm and 0.26mm of marginal bone loss was observed at 8 and 12 weeks respectively. The micro-threaded 8 and 12 week specimens. A complex soft tissue arrangement was observed against micro-threaded and micro-grooved implants.
Conclusions: This is an animal study which looked at the marginal bone level and the soft tissue reaction between different implant systems with various neck designs. Within the limitation of this animal study the following statement can be concluded;
1. A clear morphometric difference in the bone area could not be noticed between MT and MG implant neck types.
2. The BIC in MG implants were slightly higher than corresponding healing times of MT and TN implants. Higher values of the BIC could be measured in week 12 specimens than in week 8 specimens.
3. In the marginal bone level, there was marked lowering with the TN implants and least with MG implants from the reference point. There were higher marginal bone levels in week 12 than week 8 in MT and MG implants specimens but with minimal differences in TN implant specimens.
4. With MT and MG implant surfaces, the collagen alignments were not parallel to the long axis of the implants. The MT and MG implants, especially MG implants had advantageous tissue response in comparison to the turned neck implants.
ABSTRACT
Purpose: The purpose of this study was to examine the crestal bone, connective tissue, and epithelial cell response to a laser microtextured collar compared with a machined collar, in the dog model.
Materials: Six mongrel dogs had mandibular premolars and first molars extracted and after healing replaced with BioLok implants 4x8 mm. Each dog had 3 control implants placed on one side of the mandible and 3 experimental, laser microtextured, implants placed contralaterally. After 3 months, 1 dog was killed. Bridges were placed on the implants of 4 of the dogs. The sixth dog served as a negative control for the duration of the experiment. Two of the dogs were killed 3 months after loading, two of the dogs were killed 6 months after loading as was the negative (unloaded) control. Histology, electron microscopy, and histomorphometric analysis was done on histologic sections obtained from block sections of the mandible containing the implants.
Results: Initially the experimental implants showed greater bone attachment along the collar. With time the bone heights along the control and experimental collars were equivalent. However, the controls had more soft tissue downgrowth, greater osteoclastic activity, and increased saucerization compared with sites adjacent to experimental implants. There was closer adaptation of the bone to the laser microtextured collars.
Conclusions: Use of tissue engineered collars with microgrooving seems to promote bone and soft tissue attachment along the collar and facilitate development of a biological width.
ABSTRACT
It has been shown that implant designs and different vertical positions have an influence on crestal bone. The purpose of this study was to use finite element (FE) analysis to biomechanically investigate the influence of the stress/strain distribution in a maxillary anterior 3.0-mm-diameter implant in relation to its apicocoronal level after oblique loading. Two different FE models, depending on implant position relative to bone crest, were applied. It can be concluded that placing the implant-abutment interface sub-crestally provides decreased levels of stress and strain in the surrounding bone. However, placing the implant 0.5 mm supracrestally is also acceptable according to this analysis.
ABSTRACT
Surface microgeometry plays a role in tissue implant surface interactions, but our understanding of its effects is incomplete. Substrate microgrooves strongly influence cells in vitro, as evidenced by contact guidance and cell alignment. We studied "dot" colonies of primary fibroblasts and bone marrow cells that were grown on titanium-coated, microgrooved polystyrene surfaces that we designed and produced. Rat tendon fibroblast and rat bone marrow colony growth and migration varied (p < 0.01) by microgroove dimension and slightly by cell type. We observed profoundly altered morphologies, reduced growth rates, and directional growth in colonies grown on microgrooved substrates, when compared with colonies grown on flat, control surfaces (p < 0.01). The cells in our colonies grown on microgrooved surfaces were well aligned and elongated in the direction parallel to the grooves and colonies. Our "dot" colony is an easily reproduced, easily measured and artificial explant model of tissue implant interactions that better approximates in vivo implant responses than culturing isolated cells on biomaterials. Our results correlate well with in vivo studies of titanium dioxide-coated polystyrene, titanium, and titanium alloy implants with controlled microgeometries. Microgrooves and other surface features appear to directionally or spatially organize cells and matrix molecules in ways that contribute to improved stabilization and osseointegration of implants.
ABSTRACT
Orthopedic implants often loosen due to the invasion of fibrous tissue. The aim of this study was to devise a novel implant surface that would speed healing adjacent to the surface, and create a stable interface for bone integration, by using a chemoattractant for bone precursor cells, and by controlling tissue migration at implant surfaces via specific surface microgeometry design. Experimental surfaces were tested in a canine implantable chamber that simulates the intramedullary bone response around total joint implants. Titanium and alloy surfaces were prepared with specific microgeometries, designed to optimize tissue attachment and control fibrous encapsulation. TGFβ, a mitogen and chemoattractant (Hunziker EB, Rosenberg LC. J Bone Joint Surg Am 1996;78:721-733) for osteoprogenitor cells, was used to recruit progenitor cells to the implant surface and to enhance their proliferation. Calcium sulfate hemihydrate (CS) was the delivery vehicle for TGFβ; CS resorbs rapidly and appears to be osteoconductive. Animals were sacrificed at 6 and 12 weeks postoperatively. Results indicated that TGFβ can be reliably released in an active form from a calcium sulfate carrier in vivo. The growth factor had a significant effect on bone ingrowth into implant channels at an early time period, although this effect was not seen with higher doses at later periods. Adjustment of dosage should render TGFß more potent at later time periods. Calcium sulfate treatment without TGFβ resulted in a significant increase in bone ingrowth throughout the 12-week time period studied. Bone response to the microgrooved surfaces was dramatic, causing greater ingrowth in 9 of the 12 experimental conditions. Microgrooves also enhanced the mechanical strength of CS-coated specimens. The grooved surface was able to control the direction of ingrowth. This surface treatment may result in a clinically valuable implant design to induce rapid ingrowth and a strong bone-implant interface, contributing to implant longevity.
ABSTRACT
This paper presents the results of an experimental study of the interactions between MC3T3-E1 (mouse calvarian) cells and textured Ti6Al4V surfaces, including surfaces produced by laser microgrooving; blasting with alumina particles; and polishing. The multiscale interactions between MC3T3-E1 cells and these textured surfaces are studied using a combination of optical scanning transmission electron microscopy and atomic force microscopy. The potential cytotoxic effects of microchemistry on cell-surface interactions also are considered in studies of cell spreading and orientation over 9-day periods. These studies show that cells on microgrooved Ti6Al4V geometries that are 8 or 12 micron deep undergo contact guidance and limited cell spreading. Similar contact guidance is observed on the surfaces of diamond-polished surfaces on which nanoscale grooves are formed due to the scratching that occurs during polishing. In contrast, random cell orientations are observed on alumina-blasted Ti6Al4V surfaces. The possible effects of surface topography are discussed for scar-tissue formation and improved cell surface integration.
April 24-27, 2002. Tampa, FL.
ABSTRACT
Introduction: This report describes the use of laser-microtextured transcutaneous implants in a rabbit calvarial model to enhance soft tissue integration. Dental and orthopaedic implants are routinely microtextured to enhance tissue integration. Computer-controlled laser microtexturing techniques that produce microgrooved surfaces with defined 8-12μm features on controlled regions of implant surfaces have been developed based on results from cell culture experiments and in vivo models. These textures have been replicated onto the collars of dental implants to provide specific areas for both osteointegration and the formation of a stable soft tissue-implant interface. The objective of this study is to evaluate these implants in a transcutaneous rabbit calvarial model to determine whether controlled laser microtexturing can be used to create a stable interface with connective tissue and epithelium.
Methods: Laser microtextures were produced on the 4mm diameter collars of modified dental implants designed for rabbit studies (Figure 1). The implants were 4.5mm in length and the threaded portion was 3.75mm in diameter. Implants were produced and supplied by Orthogen Corporation (Springfield, NJ) and BioLok International (Deerfield Beach, FL). The implant surfaces were modified by ablation of defined areas, using an Excimer laser and large-area masking techniques. Controlled laser ablation allows accurate fabrication of defined surface microstructure with resolution in the micron scale range. Laser machined surfaces contained 8μm and 12μm microgrooved systems oriented circumferentially on the collars. The collars of the control implants were "as machined", and were characterized by small machining marks on their surfaces. All implants were cleaned and passivated in nitric acid prior to sterilization.
Four transcutaneous implants were surgically implanted bilaterally in the parietal bones in each rabbit using single-stage procedures. The surgical protocol was similar to dental implant placement. An incision was made over the sagittal suture, and the skin and soft tissues were reflected laterally. Implants were placed using pilot drills and fluted spade drills to produce 3.4mm sites for the 3.75mm diameter implants. The implants were placed with the threaded portion in bone, and the laser-microtextured collar penetrating the subcutaneous soft tissue and epithelium. Each rabbit received two implants on either side of the midline (1 control and 3 experimental implants per subject). The skin was then sutured over the implants. Punch openings were made to expose the tops of the platforms of the implants, and the cover screws were used to fasten down small plastic washers coated with triple antibiotic ointment. The plastic washers were used to prevent the skin from closing over the implant during the swelling that occurred during early healing. They were removed after two weeks. Twelve rabbits were used in the study. Rabbits were sacrificed at 2, 4 and 8 weeks, and the implants and surrounding tissues were processed for histology. Hard and soft tissue response to the implants was examined histologically.
Results and Discussion: No complications or infections were encountered during the course of the experiment. The 2 and 4 week histology displayed immature soft tissue formations around all implants, and little epithelial interaction with the implant surfaces was noted as the epithelium had not regenerated at the implant surface by 2 weeks, and no clear relationship between epithelium and implant was seen at 4 weeks. 8-week samples showed more mature soft tissue and epithelial tissue. In these samples, the epithelium had fully regenerated and the soft tissue showed more mature and organized collagen. In the control samples, the epithelium consistently grew down the interface between implant and soft tissue and formed a deep sulcus along the implant collar. This sulcus extended to the bone surface and there was little or no direct soft tissue interaction or integration with the control surfaces. The 8-week laser machined implants produced a different pattern of tissue interaction. The epithelium also produced a sulcus at the upper collars of these implants. However, in most cases the sulcus did not extend down as far as the bone surface, but ended at a 300-700μm wide band of tissue, which was attached to the base of the microtextured collar. Even though the laser-microtexturing extended to the top of the collar, this soft tissue attachment formed only at the lower portion of the implant collar, where a stable "corner" of soft tissue attached to both the implant collar and the bone surface. This arrangement of sulcus, epithelial attachment, and soft tissue attachment was similar to the "biologic width" structural arrangement that has been described around teeth and in some cases around implants.
Conclusions: This preliminary study suggests that laser microtextured surfaces can be applied to transcutaneous implants and used to improve soft tissue integration. Results suggested that the soft tissues at the skin interface are capable of producing an arrangement similar to the "biologic width" arrangement seen around teeth. These laser-machined microtextures are hypothesized to work by increasing surface and organization of attached cells and tissues. They can be used to form a functionally stable interface with soft tissues, establishing an effective transcutaneous barrier. While longer-term studies are needed, the results suggest that performance of transcutaneous prosthetic fixation may be enhanced through the use of regional organized microtexturing.
Published by Em2 Inc., Toronto, Canada. 2000.
INTRODUCTION
Tissue response to any implantable device has been found to correlate with a complex combination of material interface parameters based on composition, surface chemistry, and surface microgeometry. The relative contributions of these factors are difficult to assess.
In vitro and in vivo experiments have demonstrated the role of surface microgeometry in tissue-implant surface interaction although no well-defined relationship has been established. The general relationship, as demonstrated by in vivo experiments on metallic and ceramic implants indicates that smooth surfaces promote formation of thick fibrous tissue encapsulation and rough surfaces promote thinner soft tissue encapsulation and more intimate bone integration. Smooth and porous titanium surfaces have also been shown to have different effects on the orientation of fibrous tissue cells in vitro. Surface roughness has been shown to be a factor in tissue integration of implants with hydroxyapatite surfaces, and to alter cell attachment and growth on polymer surfaces roughened by hydrolytic etching. Roughened surfaces have also shown pronounced effects on differentiation and regulatory factor production of bone cells in vitro. Defined surface microgeometries, such as grooved and machined metals and polymer surfaces have been shown to cause cell and ECM orientation in vivo and can be used to encourage or impede epithelial downgrowth in experimental dental implants. Surface texturing has also been shown to adhere fibrin clot matrix more effectively than smooth surfaces, forming a more stable interface during the collagenous matrix contracture that occurs during healing. This is an effect which may be important in determining early events in tissue integration.
It is likely that textured surfaces work on several levels. These surfaces have higher surface areas than smooth surfaces and interdigitate with tissue in such a way as to create a more stable mechanical interface. They may also have significant effects on adhesion of fibrin clot, adhesion of more permanent extracellular matrix components, and long-term interaction of cells at stable interfaces. We have observed that, in the short term, fibrous tissue cells form an earlier and more organized collagenous capsule at smooth interfaces than at textured interfaces. We suggest that textured surfaces have an additional advantage over smooth surfaces. They inhibit colonization by fibroblastic cell types that arrive early in wound healing and encapsulate smooth substrates.
We have investigated (1) the effects of textured surfaces on colony formation by fibroblasts, and (2) the effects of controlled surface microgeometries on fibroblast colonization. Based on these results, we have designed, fabricated, and tested titanium alloy and commercially-pure titanium implants with controlled microgeometries in in vivo models. These experimental surfaces have highly-oriented, consistent microstructures which are applied using computer-controlled laser ablation techniques. The results suggest that controlled surface microgeometry, in specific size ranges, can enhance bone integration and control the local microstructural geometry of attached bone.
May 15-20, 2000.
ABSTRACT
Introduction: Implant surface geometry and microgeometry influence tissue responses to implants. The physical and chemical properties of synthetic substrates affect the morphology, physiology and behavior of cultured cells of various types. To date, studies of tissue-implant interaction have emphasized cell attachment, signaling and other cellular response mechanisms. Cellular attributes influenced by micrometric substrate features include cell shape, attachment, migration, orientation, and cytoskeletal organization. We studied three phenotypic variants of a murine fibroblast cell line to explore the influence of substrate microgeometry on cell shape, orientation, and microfilament distribution. Microfilament organization reflects cell shape and orientation, attendant to cell signaling events that also regulate cell attachment, mitosis, migration and apoptosis. Microfilament bundles (stress fibers) terminate at clusters of actin-associated proteins, adhesion molecules, and protein kinases which contribute to in vitro cell responses to culture substrates.
Methodology: NIH-3T3 fibroblasts, 3T3-Li fibroblasts (ATCC, Manassas, VA) and MC-3T3 fibroblasts (gift of JP O'Connor) were grown in DMEM with 10% NCS and 1% antibiotics in 24-well plates containing TiO2-coated, microtextured polystyrene inserts. Culture substrates had either 8μm parallel grooves, 12μm parallel grooves, 3x3μm square posts separated by 3μm perpendicular grooves, or no features (controls). Ten thousand cells were seeded into wells containing the inserts and after 1 day, were prepared for scanning electron microscopy (SEM) or stained with rhodamine-phalloidin.
Results: All three variants of 3T3 fibroblasts adhered to all substrates within 1 day. There was no predominant orientation or shape in cells grown on control surfaces. The cytoplasms of some cells grown on control surfaces showed random arrays of stress fiber, apparently terminating at focal adhesions. Nearly all cells of all types grown on 8 or 12μm grooved substrates were elongated and oriented parallel to the grooves, growing atop the ridges or within the troughs (Figure 1). Cells cultured on 8μm grooves bridged grooves more frequently than cells cultured on 12μm grooves. Few cells of any type demonstrated evidence of stress fibers formation after 1 day in culture on grooved surfaces. Many cells grown on posted substrates displayed stress fibers terminating on posts. These assumed a stellate conformation, with process extending orthogonally from a central cytoplasmic mass and terminating atop the elevated posts (Figure 1). This finding is similar to our previous observations of NIH-3T3 cells grown on posted substrates. SEM observations confirmed the shape and orientation effects of the substrates on the 3T3 variants.
Discussion: This experiment demonstrated that parallel and intersecting grooves dictate the cell shape, orientation and cytoskeletal organization of three phenotypic variants of 3T3 fibroblasts. The NIH-3T3 variant is a fibrogenic line, while the 3T3-L1 variant is lipogenic and the MC-3T3 variant is osteogenic. The phenotypes of these cells were assessed by alkaline phosphatase assay (MC-3T3 cells are alkaline phosphatase positive) and by Sudan Black B staining (for lipid inclusions in 3T3-Li cells). The roles of extracellular matrix and cell adhesion molecules in the contact guidance events described above were not characterized, but are not discounted. We have previously demonstrated that integrin distribution and tyrosine kinase activity is physically constrained by micrometric substrate features. We hypothesize that the same constraint has occurred in the cells described herein. Elucidation of phenotypic differences between cell types that direct the tissue response to implants may yield information leading to improved implant integration and extended implant lifespan.
Acknowledgements: This work was aided by NSF grants SBIR-9160684 and DUE-9750533, and by NJCU SBR grant 220253. Microgeometry molds were prepared by the Cornell Nanofabrication Facility.
April 22-26, 1998. San Diego, CA.
ABSTRACT
Introduction: Implant surface geometry and microgeometry affect tissue responses, although the tissue-implant interaction is incompletely characterized. Physical and chemical properties of synthetic substrates affect the morphology, physiology and behavior of cultured cells of various types. Investigators are just now beginning to describe these in vitro effects in detail. Shape, attachment, migration, orientation, and cytoskeletal organization differ between cells cultured on flat substrates and substrates having regular surface features of micrometric dimensions. We studied murine fibroblast shape, orientation, and microfilament and focal adhesion distribution - parameters relevant to contact guidance and to other factors influenced by substrate microgeometry. Microfilament organization reflects cell shape and orientation, but is also important in signal transduction schemes governing cell attachment, mitosis, migration and apoptosis. Microfilament bundles terminate in clusters of actin-associated proteins, adhesion proteins, and protein kinases having signal transduction functions. We employed assays that revealed the distribution of (1) microfilaments/stress fibers; (2) focal adhesion molecules; and (3) phosphotyrosine, the product of the major class of kinases associated with cell attachments.
Methodology: 3T3 fibroblasts (ATCC, Rockville, MD) from frozen stocks were grown in DMEM with 10% FBS in multiwell plates containing 1cm square microtextured inserts. The inserts consisted of polystyrene solvent cast on silicon molds and titanium-oxide coated. The resultant surfaces had either 8μm parallel grooves, 12μm parallel grooves, 3μm square posts (created by perpendicular 3μm grooves), or no features (controls). Four thousand cells were seeded into wells containing the inserts and after 4 or 8 days were prepared for scanning electron microscopy (SEM) or stained with (1) rhodamine-phalloidin; (2) either mouse antitalin or mouse antivinculin followed by rhodamine-antimouse antibodies; or (3) fluorescein-antiphosphotyrosine antibody.
Results: By day 4, the 3T3 cells had adhered to all substrates, and by day 8 they showed considerable growth in places approaching confluences. There was no predominant orientation or shape in cells grown on control surfaces. Their cytoplasms showed diffuse rhodamine staining; demonstrable stress fibers were absent. Focal adhesions and phosphotyrosine were diffusely distributed. Cells grown on 8 or 12μm grooved substrates were nearly uniformly oriented in the direction of the grooves. Cells cultured on 8μm grooves mostly grew atop the ridges, often bridging the troughs between ridges. Cells cultured on 12μm grooves mostly grew either atop the ridges or within the troughs, only infrequently bridging the troughs between ridges. Some cells demonstrated limited evidence of stress fibers after 8 days in culture on the grooved surfaces. Focal adhesions and phosphotyrosine were limited to areas of cell-substrate contact; portions of cells spanning troughs lacked focal adhesions and phosphotyrosine. Cells grown on the posted surfaces showed orthogonal arrays of microfilaments that conformed to the intersecting troughs between posts; stress fibers, however, were not observed. These cells either rested atop the posts or settled down onto the posts, with the posts apparently displacing cytoplasm and limiting the distribution of microfilament bundles to areas of basal contact. SEM observations confirmed that the posts penetrated the basal cell membrane surface, with the cell contents settling around the posts. Focal attachments and phosphotyrosine were similarly distributed in these cultures.
Discussion: This experiment demonstrated that parallel and intersecting grooves are capable of affecting the shape, orientation, cytoskeletal organization, and distribution of focal adhesions in 3T3 fibroblasts, extending our previous findings of these effects in rat tendon fibroblasts. The role of extracellular matrix in guiding this process, while not characterized, is not discounted. The limitation of kinase activity by physical substrate features is a novel finding and could shed light on mechanisms by which cell types respond differentially to substrates. Ultimately, we hope to uncover phenotypic differences in these properties between cell types that will direct the tissue response to implants in ways that improve the incorporation of and extend the functional life spans of the implants.
Acknowledgements: This work was aided by National Science Foundation SBIR phase I grant #9160684 and by Jersey City State College SBR grant #220251. Microgeometry molds were prepared by the Cornell Nanofabrication Facility.
April 30-May 4, 1997. New Orleans, LA.
ABSTRACT
Introduction: Surface microgeometry influences tissue-implant interaction, although the interaction is poorly understood. Cellular contact guidance, a tissue response to surface microgeometry, profoundly influences cell growth and other behaviors. For example, on grooved surfaces, groove depth and width minima are required to affect cell shape and orientation and the direction of growth. Cytoskeletal organization reflects cell attachment, shape and orientation and likely contributes to these microgeometry-directed phenomena. We examined some properties of fibroblasts cultured on simulated biomaterials with various surface microgeometries. We studied rat tendon fibroblast (RTF) cells, because human fibrous tissue cells are among the first cells to contact implants. Fibrous implant encapsulation is influenced by surface roughness and microgeometry: roughening promotes thinner capsule development and, therefore, more intimate contact of bone cells and tissue with the implant and improved implant integration.
Methodology: RTFs from stock cultures derived from hind foot extensor tendons were grown on smooth (control) and patterned polystyrene substrates having parallel 2 or 12μm linear grooves or 8x50 or 80x50μm diamond-shaped islands separated by 3x3μm grooves. Substrates were solvent-cast on silicon molds and titanium oxide coated. Fifteen millimeter circular cutouts were fitted into 24-well plates, and wells containing inserts were seeded with 20,000 RTFs and fixed after 4 and 8 days in culture. Cell morphology was studied and recorded by scanning electron microscopy and by fluorescence microscopy of cultures stained with rhodamine phalloidin and anti-vinculin followed by a fluorescein-conjugated secondary antibody.
Results: The orientations and shapes of RTFs grown on control and patterned surfaces were consistently different. Cell orientation tended to be random in control cultures, but generally coincided with the directions of the linear grooves and the longer dimensions of the diamonds. RTFs grown on 2μm grooved and diamond-patterned substrates often bridged the grooves, attaching to elevated substrates. RTFs grown on 12μm grooves grew both within the grooves and on elevated surfaces, but rarely bridged grooves. RTFs grown on the larger diamond patterned substrate often grew in clusters on elevated areas. RTFs grown on control surfaces were approximately round and symmetrical, extending short processes omnidirectionally from a central cell mass. RTFs grown on linear substrates more typically assumed a spindle shape, and extend processes perpendicular to the grooves only when spanning a narrow (2 or 3μm) groove or to establish lateral contact with groove walls (12μm grooves). Substrate microgeometry also affected the organization of microfilament bundles (stress fibers), which were aligned with the predominant direction of cellular orientation in cells grown on the patterned substrates. RTFs from control cultures typically showed microfilament bundles extending at miscellaneous angles throughout the cell cytoplasm. In all cells, vinculin was localized at microfilament bundle termini, as revealed by imunofluorescence microscopy, indicating points of cell-substrate attachment consistent with the presence of focal attachments.
Conclusions: This study showed that both the linear and diamond patterns are capable of influencing the orientation and cytoskeletal organization of fibroblast cells, extending previous observations of contact guidance effects based on substrate microgeometry on cell shape alteration and directional growth. RTFs, which vary from 3 to 10μm in width, frequently bridged 2 and 3μm grooves, suggesting that more pronounced surface features may be required to optimally control the growth of these cells. The results of this experiment differ from earlier reports of the growth of "dot" cultures prepared with cells suspended in a collagen gel. Seeded cultures appear to be less sensitive to microgeometry effects than dot cultures. Outgrowing dot culture cells probably migrate considerable distances across substrate surfaces. Grooves may thus serve as more substantial guides to migrating dot culture cells than to cells in seeded cultures, which settle and thereafter remain stationary. Continued experimentation and comparison of these models, particularly in cellular attachment to substrates, will yield additional insight into the behaviors of these cells on patterned substrates. For instance, it may prove possible to control the rate and direction of fibrous tissue growth at the tissue-implant interface, thereby optimizing the stability of these implants.
Acknowledgements: This work was aided by NSF SBIR phase I grant# 9160684. Microgeometry molds were prepared by the Cornell Nanofabrication Facility.
May 29-June 2, 1996. Toronto, Canada.
ABSTRACT
Introduction: It has long been recognized that implant surface microtexture can influence tissue interaction. In previous studies we have examined the in vitro interaction of connective tissue fibroblasts with a variety of defined surface microgeometries, including microgrooved, roughened and more complex surfaces. In most cases, these surfaces, while having similar composition, have different (and pronounced) effects on the rate and direction of growth of fibroblast cell colonies. The mechanism of surface microgeometry's effect on cell colony growth rate is unknown. This study investigated the effect of defined surface microgeometry on connective tissue cell colony density, cell attachment area (spreading), and cell shape. The results suggest a possible basic mechanism of surface microgeometry control of attached cell growth.
Materials and Methods: Rat tendon fibroblast (RTF) cells were grown as stock cultures from hindfoot extensor tendons from 14-day-old Sprague-Dawley rats. Second to fourth-passage cells, grown in Dulbecco's Modified Eagle's Medium containing penicillin-streptomycin and 10% fetal bovine serum were used for all experiments. Cell colonies were grown on these surfaces using a "dot" culture model similar to explant culture models. These cells were suspended in solubilized collagen (Vitrogen, Celltrix, Palo Alto, CA) and 2μL droplets containing 20,000 cells each were polymerized on the experimental surfaces, where they acted as sources of radiating cell colony growth. Light microscopy and image analysis methods were used to measure rate and direction of growth as well as cell density (cells/mm2), cell attachment area (μm2), cell orientation (relative to substrate orientation), and cell elongation (eccentricity, the ratio of cell length to cell width). For individual cell measurements, 30 cells of each experimental group were measured. Experimental substrates consisted of solvent-cast polystyrene surfaces, vapor-deposited with 60nm of TiO2, molded from silicon wafer templates produced by optical lithography methods at the National Nanofabrication Facility at Cornell University (Ithaca, NY). Substrates consisted of mirror-smooth surfaces (controls) and square-wave microgrooves with ridges and grooves 1.75, 6.5 and 12μm in size. Results were analyzed for statistical significance using t-tests.
Results: All three microgrooved surfaces had a pronounced effect on cell colony growth, cell attachment area, cell eccentricity, cell density, and cell orientation (Table 1): they reduced cell colony growth and cell spreading, increased cell eccentricity (elongation), and effectively oriented the cells parallel to the surface. Cell density on all experimental surfaces was reduced relative to controls.
Discussion: Well-defined surface microgeometries with the tested dimensions are effective at orienting cells, changing cell shape, and reducing cell growth rates. It is well known that attachment-dependent cells must attach and spread to trigger cell division. The present results suggest that the growth inhibition effect demonstrated by these surfaces may be based on reduction of cell spreading by the surface microgrooves. These experiments suggest that observed differences in fibrous encapsulation of smooth vs. microtextured surfaces may be based on direct suppression of fibroblast spreading and growth by microtextures. These microgeometries have potential application as implant surfaces for control of tissue integration.
Acknowledgements: This work was funded by Orthogen Corporation through NSF SBIR Phase I award 9160684.
March 18-22, 1995. San Francisco, CA.
ABSTRACT
Introduction: Soft tissue encapsulation of an implant has been found to correlate with the composition, surface chemistry, and surface microgeometry of the implant material. Surface microgeometry (or surface texture) of metal implants in bone has been shown to influence fibrous capsule formation. For example, smooth surfaces induce thicker fibrous capsule formation than roughened surfaces, suggesting that surface microgeometry influences fibrous tissue proliferation. We evaluated the in vitro response of rat tendon fibroblast (RTF) cell colonies, and human implant capsule fibroblast (HICF) cell colonies, from fibrous capsule tissue from around total hip replacement components, to surfaces roughened by blasting techniques, and to controlled surface microgeometries consisting of small square post projections with features from 3 to 12 μm in size.
Materials and Methods: RTF cells were grown from hind-foot extensor tendons of 14-day-old Sprague Dawley rats. HICF cells were grown from fibrous capsule tissue samples obtained from patients undergoing total hip revision, including removal of an uncemented hip prosthesis. The tissues, obtained from an area near the proximal stem, were grown as explants under sterile conditions to produce stock cell cultures. All cells were grown as stock cultures, mixed with solubilized collagen, and dispensed and polymerized to initiate "dot" cultures, consisting of 2μL dots each containing 20,000 cells, on all experimental surfaces. These cell-collagen dots acted as sources of cell outgrowth to form growing cell colonies. At 4 and 8 days, cell colonies were fixed, stained, and measured for area of growth using a video camera-equipped stereomicroscope connected to a computer image-processing/image-analysis system. Growth of the cell colonies was measured as area or diameter increase between 4 and 8 days. Roughened surfaces were produced by grit-blasting or bead-blasting of polystyrene culture plates. A masked area was used as a smooth control surface. These surfaces consisted of a range of feature sizes depending on blast medium. The media were similar to those used to texture metal orthopaedic implants and produced similar sized features. Controlled microgeometry substrates were molded in solvent-cast polystyrene from templates precision-fabricated using optical lithography methods at the National Nanofabrication Facility at Cornell University. All surfaces were sputter-coated with a 600-Å layer of TiO2 to simulate an orthopaedic titanium alloy implant surface. The controlled microgeometry surfaces consisted of well-characterized, cross-hatched or checkerboard surface patterns consisting of square posts in 3, 6, 10 and 12μm feature sizes.
Results: All cell colonies showed consistent growth by day 4, with cell outgrowth observed at the periphery of the dot. Randomly oriented cells formed circular colonies on the control surfaces, and the roughened surfaces. The controlled microgeometry surfaces produced colonies with unusual shapes because of surface restriction of direction of growth. On an individual cell level, the cells were observed to orient along surface structures and in grooves between surface structures. On the smallest controlled microgeometries, each cell was observed to attach to the surfaces of several of the square posts. All of the experimental surfaces significantly inhibited cell colony growth in both types of cells. Significant cell growth inhibition was observed on the grit-blasted Ti-coated surface (GB-Ti) compared to the control Ti-coated surface (C-Ti) and the control untreated culture plate (C-p) as shown in Fig. 1 which represents RTF cell growth. The most efficient cell growth inhibition was observed on the 3μm cross-hatched surface (Fig. 2), although all of the cross-hatched surfaces caused significant inhibition of colony growth. Figure 2 also shows RTF cell colony data.
Conclusion: RTF and HICF cell colonies grown on roughened surfaces and on a series of controlled microgeometries exhibited pronounced inhibition of growth. The surfaces did not increase cell density in the colonies and the effect was not based on increased substrate surface area. The observed results represent the effects of cellular contact guidance - the ability of substrate microgeometry to influence cell orientation and migration - on overall growth of cell colonies. The observed effects of roughened surfaces and microgeometries on fibrous tissue cell growth, in vitro, may be related to the observation that roughened surfaces cause less fibrous encapsulation in vivo. If so, controlled microgeometry surfaces may be used to effectively suppress fibrous encapsulation.
Acknowledgements: This work was supported by Orthogen Inc., and was aided by a grant from the Orthopaedic Research and Educational Foundation.
ABSTRACT
Where does dentistry fit into the field of regenerative medicine? Based on the fact that the goal of regenerative medicine is to restore function to damaged organs and tissues, it is apparent that dentistry, which has long embraced the concept of restoring function of damaged teeth, has embraced this goal from the very beginning. In this brief review we present the opinion that if you take as the primary criterion the restoration of tissue and organ function, dentistry has not only been at the forefront of restorative medicine but actually predates it in practice. We illustrate the depth and breadth of dental regenerative medicine using examples of therapies or potential therapies from our laboratories. These begin with an example from a historical area of strength, dental implant design and fabrication, progress to a more high tech bone scaffold fabrication project, and finish with a stem cell-based soft tissue engineering project. In the final analysis we believe that the restorative nature of dentistry will keep it at the forefront of regenerative medicine.
Surface engineered dental implants – regenerative medicine in clinical use
Dental implants have become a popular and successful approach to restoration of function of lost teeth. Their success is based on their ability to integrate in bone and soft tissue, although the importance of soft tissue integration, until recently, has not been recognised or adequately addressed. Since dental implants are one of the few medical devices that are permanent and transcutaneous, integration of epithelium and fibrous connective tissue are important to form a seal against the oral environment.
Clinical application of laser micromachined surfaces
Putting grooves on implants is not new in implant dentistry and there are a number of high quality implants in the field that have micro threaded collars. However, these are not identical to Laser-Lok. They were not engineered with regenerative medicine in mind, and the grooves are not in the same order of magnitude as the very small 8–12µm channels on the Laser-Lok surface as they were not designed to act on a cellular level. For this reason, the other implants do not affect cellular behaviour to the same level. The Laser-Lok implant design is based on regenerative medicine concepts that have been highly successful and has led to better regeneration of the soft tissue and bone around the restoration. This has changed the paradigm of implant surface technology, and demonstrated that cell and tissue response can be controlled at the implant interface, using regenerative medicine-based concepts.








